The history of the atom worksheet answers unfolds a captivating tale, tracing the evolution of our understanding of the atom from its ancient roots to the present day. This journey reveals the groundbreaking contributions of brilliant scientists and the pivotal discoveries that have shaped our knowledge of the fundamental building blocks of matter.
From Democritus’s early speculations to Rutherford’s groundbreaking experiments, the pursuit of unraveling the atom’s secrets has been a relentless endeavor. This worksheet provides a comprehensive exploration of this scientific odyssey, offering insights into the structure, properties, and applications of atoms, while highlighting the ethical considerations that accompany their harnessing.
History of the Atom
The history of the atom dates back to ancient Greece, where philosophers proposed that matter was composed of indivisible particles called atoms. Over the centuries, scientists have refined and expanded our understanding of the atom, leading to the modern atomic model.
Key scientists and their contributions to the understanding of the atom include:
- Democritus (c. 460-370 BC): Proposed the idea of atoms as indivisible particles.
- John Dalton (1766-1844): Developed the atomic theory, which stated that all matter is composed of atoms, which are indivisible and indestructible.
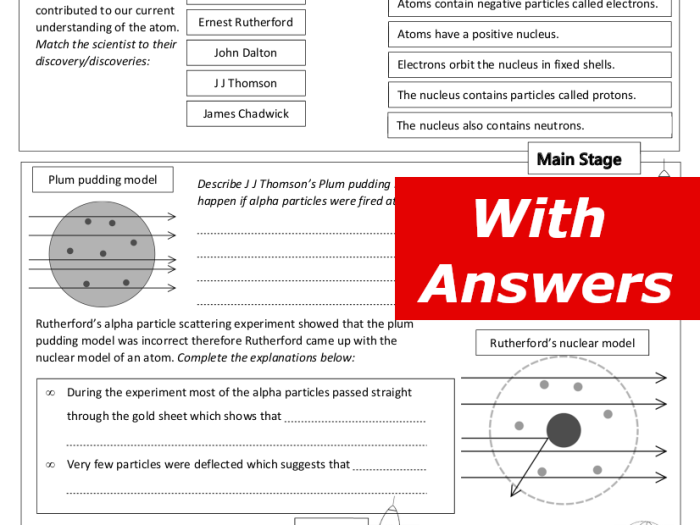
- J.J. Thomson (1856-1940): Discovered the electron, which led to the development of the plum pudding model of the atom.
- Ernest Rutherford (1871-1937): Discovered the nucleus of the atom, which led to the development of the nuclear model of the atom.
- Niels Bohr (1885-1962): Proposed the Bohr model of the atom, which explained the energy levels of electrons.
- Erwin Schrödinger (1887-1961): Developed the quantum mechanical model of the atom, which is the modern understanding of the atom.
Significant discoveries in atomic physics include:
- 1808: Dalton publishes his atomic theory.
- 1897: Thomson discovers the electron.
- 1911: Rutherford discovers the nucleus of the atom.
- 1913: Bohr proposes the Bohr model of the atom.
- 1926: Schrödinger develops the quantum mechanical model of the atom.
Atomic Structure
An atom is the smallest unit of matter that retains the chemical properties of an element. It consists of a nucleus and an electron cloud.
The nucleus is located at the center of the atom and contains protons and neutrons. Protons are positively charged particles, while neutrons are neutral particles. The number of protons in an atom determines its atomic number, which identifies the element.
The electron cloud surrounds the nucleus and contains electrons. Electrons are negatively charged particles. The number of electrons in an atom is equal to the number of protons, making the atom electrically neutral.
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. For example, carbon-12, carbon-13, and carbon-14 are all isotopes of carbon.
Atomic Properties

The chemical and physical properties of atoms are determined by the number of protons, neutrons, and electrons.
The number of protons determines the atomic number, which identifies the element. The number of neutrons determines the mass number, which is the total number of protons and neutrons in the nucleus.
The number of electrons determines the chemical properties of the atom. Atoms with the same number of electrons have similar chemical properties.
For example, sodium has 11 protons, 11 electrons, and 12 neutrons. This gives it an atomic number of 11 and a mass number of 23. Sodium is a highly reactive metal that reacts easily with water to form sodium hydroxide.
Nuclear Reactions: History Of The Atom Worksheet Answers
Nuclear reactions are reactions that involve the nuclei of atoms. There are two main types of nuclear reactions: fission and fusion.
Fission is a nuclear reaction in which a heavy nucleus splits into two or more lighter nuclei. This process releases a great amount of energy.
Fusion is a nuclear reaction in which two or more light nuclei combine to form a heavier nucleus. This process also releases a great amount of energy.
Nuclear reactions are used in power generation and medicine. Nuclear power plants use fission to generate electricity. Nuclear medicine uses radioactive isotopes to diagnose and treat diseases.
Applications of Atomic Physics
Atomic physics is used in a variety of fields, including medicine, materials science, and energy production.
In medicine, atomic physics is used to develop new imaging techniques, such as MRI and PET scans. Atomic physics is also used to develop new treatments for cancer, such as radiation therapy.
In materials science, atomic physics is used to develop new materials with improved properties, such as strength, durability, and conductivity. Atomic physics is also used to develop new methods for manufacturing materials.
In energy production, atomic physics is used to develop new energy sources, such as nuclear fusion. Atomic physics is also used to develop new methods for storing energy.
FAQ Compilation
What is the significance of the Bohr model of the atom?
The Bohr model introduced the concept of quantized energy levels, revolutionizing our understanding of atomic structure and laying the foundation for quantum mechanics.
How did the discovery of the neutron impact our understanding of the atom?
The discovery of the neutron explained the existence of isotopes and provided a more complete picture of the atomic nucleus.
What are the practical applications of nuclear reactions?
Nuclear reactions are harnessed in nuclear power plants to generate electricity and in medical treatments like radiation therapy.
